Introduction1-5
For many patients with knee osteoarthritis, conservative therapies like non-steroidal anti-inflammatory drugs, exercise-based therapies, and knee bracing can be effective in the initial stages. However, despite these measures, many knee arthritis patients eventually have persistent knee pain affecting their daily activities. Surgical options like total knee arthroplasty (TKA) are effective but have unsuitable risks for some patients. Side effects and contraindications further limit the few medication options.
So, what alternative can we offer to patients seeking relief?
Viscosupplementation with high molecular weight hyaluronic acid (HA) injections are increasingly recognized as a valuable non-surgical option for managing knee osteoarthritis. A 2019 meta-analysis identified HA injections as the most effective non-surgical therapy for knee osteoarthritis, outperforming other treatments in both efficacy and duration of relief. Notably, HA is the only therapy above the threshold of clinical relevance (Figure 1).
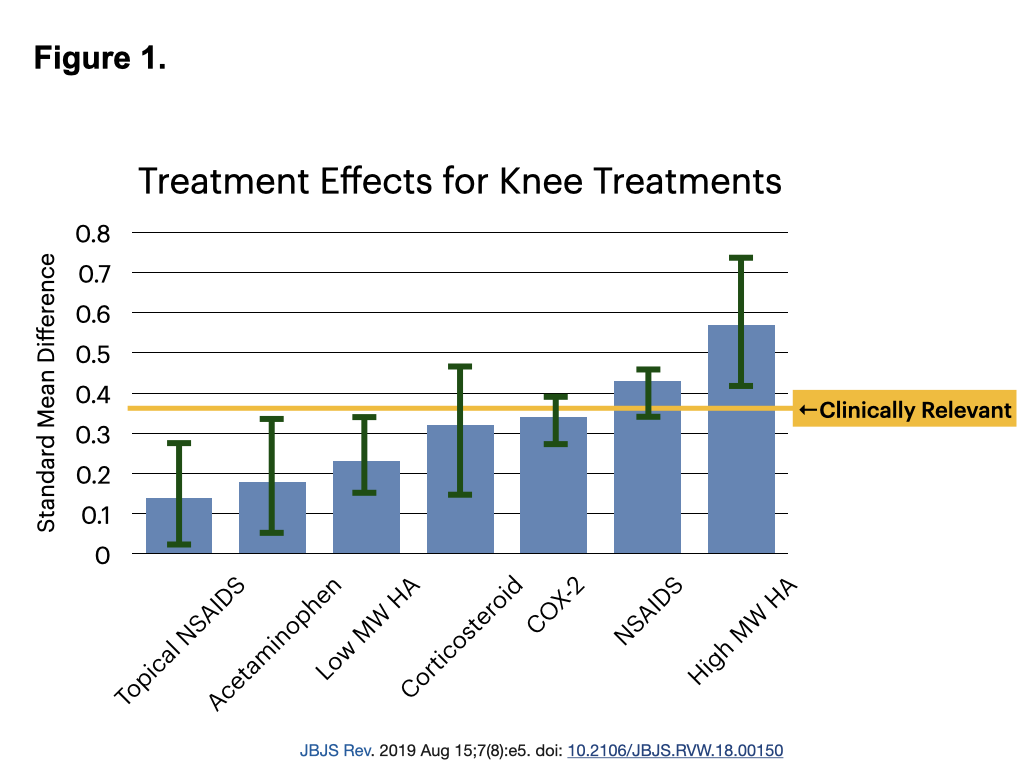
This article explores the role of HA viscosupplementation in knee osteoarthritis management, covering its mechanism of action, benefits, patient selection, comparison with glucocorticoid injections, considerations for surgery, controversies, insurance issues, and optimal injection methods.
Hyaluronic Acid Mechanism of Action6
Hyaluronic acid is a critical component of human synovial fluid, responsible for lubrication, shock absorption, and the suppression of inflammatory mediators. In osteoarthritis, the native HA lose molecular weight and concentration, increasing joint friction and inflammation. Restoring hyaluronic acid through injections provides chondroprotection, cartilage matrix synthesis, reduction of inflammation, mechanical cushioning, analgesia, and alterations in the subchondral bone. Unlike glucocorticoids, hyaluronic acid actively supports joint health, promoting healing rather than contributing to further deterioration.
HA Injections vs. Glucocorticoid Injections7-20
Efficacy and Duration of Relief
Glucocorticoid Injections:
- Offer rapid short-term pain relief, typically fading after 3 to 12 weeks.
- Widely accepted, often used, with decades of literature.
- Low cost: steroid prescription is covered by Ontario Drug Benefit (ODB), and the Ontario Health Insurance Program (OHIP) covers the procedure fee of the injection.
- Glucocorticoids show no significant improvement compared to placebo at six months.
Hyaluronic Acid Injections:
- Provide long-lasting relief, with benefits persisting from 4 to 26 weeks post-injection.
- Repeated injections enhance and prolong therapeutic effects.
- They do not contribute to cartilage degradation but promote cartilage health.
Safety Profile
Glucocorticoid Risks:
- Local Side Effects: Skin hypopigmentation, fat atrophy, tendon/ligament tears.
- Repeated glucocorticoid injections can accelerate cartilage damage.
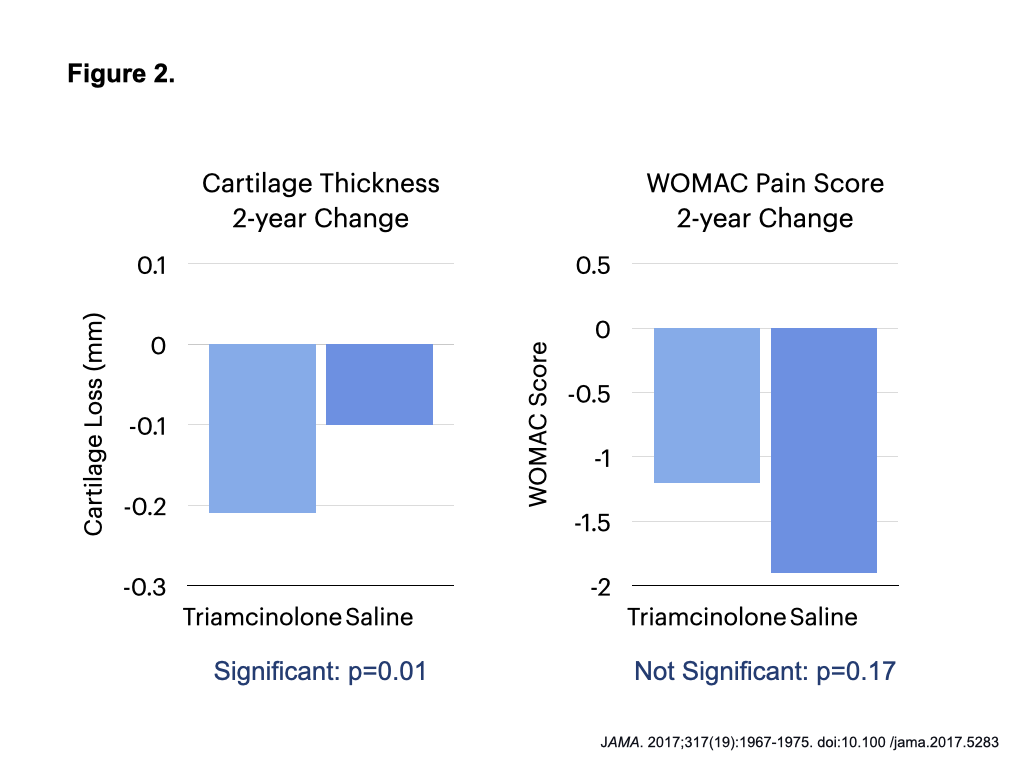
- A JAMA study, in which knees were injected with saline or triamcinolone every 6 months for 2 years, showed more cartilage loss without significant pain change for the glucocorticoid group (Figure 2).13
- Systemic Effects: Adrenal insufficiency, hyperglycemia, infection risk.
- Drug Interactions: Warfarin, Cardiac Glycosides, Immune Checkpoint Inhibitors, Loop Diuretics, Salicylates/NSAIDs
- Disease Interactions: Psychosis/Mania, Hypertension, Diabetes, Ulcers, Diverticulosis, Cirrhosis, Myasthenia Gravis, Glaucoma, Osteoporosis, Renal Disease, Seizures.
- Surgical Complications: Associated with worsened outcomes in knee replacement surgeries done within 6 months of injection.17
Hyaluronic Acid Advantages:
- Minimal Adverse Effects: In a meta-analysis of 9214 participants, local reactions, such as swelling or pain, occurred in 8.5% of HA procedures. These reactions subsided rapidly without treatment. Injection site reactions are more common in avian-derived hyaluronic acid versus biologically synthesized hyaluronic acid (13% vs 3%).
- Severe Adverse Events: Severe systemic reactions, such as infection, are present less than 0.1% of the time.
- No Major Disease Contraindications or Drug Interactions: Allows for broader patient eligibility.
- Safe for Repeated Use: Does not damage cartilage or soft tissues, enabling ongoing repeated use without added risks.
Delaying or Avoiding Total Knee Replacement21-27
The Importance of Timing in Surgery
Total knee arthroplasty is a standard procedure in severe osteoarthritis. However, not all patients will accept the risks:
- Serious Adverse Events: Occur in 1 out of 20 patients, including infections, blood clots, or cardiac complications.
- Patient Satisfaction: Approximately 1 in 5 patients express dissatisfaction after surgery due to persistent pain or complications.
- Residual Symptoms: Up to one-third may experience ongoing pain and stiffness over a year after surgery; half may struggle with limping, stair navigation, or returning to preferred activities even after 1 to 4 years of rehab.
- Recovery Time: Returning to baseline function can take 3 to 6 months, impacting work and caregiving responsibilities.
- Revision Surgery: Up to 35% of men aged 50–54 may require revision surgery within 10 years, emphasizing the importance of delaying TKA in younger patients.
Role of HA Injections in Delaying Surgery28-30
Benefits of Repeated HA Injections:
- Sustained Relief: Patients reported continued HA benefits with each additional injection for up to eight injections, each lasting 9 to 15 months.
- Reduced Surgery Rates: Studies show decreased knee replacement rates and delayed timing for TKA by at least three years when using HA.
- Quality of Life: Allows patients to maintain an active lifestyle without the risks and downtime associated with surgery.
By offering a safe and effective alternative, HA injections can fill the treatment gap for some patients who are not ready or eligible for surgery.
Clinical Considerations
Patient Selection31
- Ideal candidates for HA are patients with mild or moderate knee osteoarthritis wishing to trial non-surgical options with minimal downtime.
- HA can also be trialled in patients with severe knee osteoarthritis who must avoid surgery and are unresponsive to other conservative therapies.
Injection Techniques32
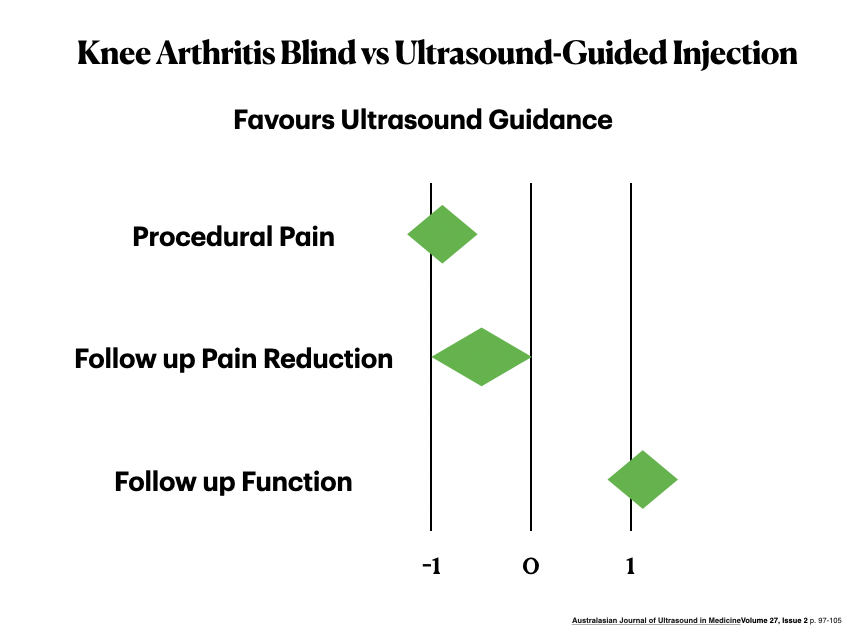
Ultrasound-guided injections are the best way to deliver HA therapy for knees.
- Higher Accuracy and improved outcomes: Meta-analysis demonstrates higher accuracy, less procedural pain, less follow-up pain and improved function with ultrasound.
Combination Therapy33
- For Immediate and Lasting Relief: Combining glucocorticoid with HA provides rapid pain relief from the steroid, followed by HA’s sustained benefits. The Canadian combination product uses triamcinolone hexacetonide, a well-tolerated steroid widely used in pediatric rheumatology. However, standard glucocorticoid precautions continue to apply.
- Clinical Evidence: A randomized trial showed a 70% pain reduction within three weeks, increasing slightly to 72% by 26 weeks, with 95% of patients reporting no adverse effects.
Addressing Controversies and Guidelines34-39
International and National guidelines, systematic reviews, and meta-analyses on HA injections for knee osteoarthritis have provided differing recommendations.
🇨🇦 The Arthroscopy Association of Canada (a subspecialty of the Canadian Orthopedic Association) gives a Grade A recommendation for intra-articular high molecular weight HA for mild to moderate knee OA.37
🇺🇸 The American Association of Orthopedic Surgeons (2019) does not recommend Hyaluronic Acid injections for knee arthritis, while the American Medical Society for Sports Medicine recommends the therapy (2015). American College of Rheumatology/Arthritis Foundation recommends limited use of HA only if other therapies have failed (2020). 🇺🇳The latest Osteoarthritis Research Society International (OARSI) guidelines recommended HA with caution (2019). 🇪🇺The European League Against Rheumatism recommended HA in 2020.
These are the methodological explanations of the contrasting conclusions:
- HA have varying formulations: Analyzing high and low molecular weight HA together diluted the efficacy of high MW HA.
- Overlooking Long-Term Benefits: Guidelines often emphasize short-term outcomes of 4-12 weeks, undervaluing HA’s sustained effects over 6 months to 3 years.
- Inconsistent Metrics: Use of non-validated statistical measures like the Minimum Clinically Important Difference (MCID) instead of Standardized Mean Difference (SMD), underestimating HA’s true impact.
- Selective Evidence Review: Exclusion of specific trials showing a benefit with HA
- Heterogenous Comparators: Treatments compared against no therapy, topical control, oral control, and intraarticular control without accounting for variations among these comparators.11
These inconsistent guidelines have led to variable insurance coverage and hesitancy in clinical adoption despite evidence that HA is a valuable option in a conservative treatment plan.
Insurance Coverage Concerns39,40
- Current Coverage: Many third-party insurers and worker’s compensation plans cover HA products. In Ontario, the HA injection procedure is not OHIP-covered and the treatment is not covered under Ontario Drug Benefit.
- Economic Considerations: HA injections are cost-effective, reducing long-term healthcare expenses by delaying surgery and decreasing daily medication use.
- Advocacy for Coverage: There’s a compelling case for public insurance to include HA injections to improve patient access and outcomes, ultimately lowering healthcare costs.
Arthroscopic Surgery Considerations41-44
- Traumatic Meniscal Tears with Mechanical Symptoms: In patients without underlying osteoarthritis, arthroscopic surgery followed by HA injections leads to better outcomes than surgery alone.
- Degenerative Meniscal Tears: In patients with osteoarthritis and degenerative meniscus tears, arthroscopic surgery showed no benefit over sham surgery or conservative management. HA injection is a non-surgical alternative, with clinical promise in one trial in degenerative tears.41
Conclusion
High molecular weight hyaluronic acid injections represent a significant advancement in the non-surgical management of knee osteoarthritis:
- Effective: Provides clinically significant pain relief and functional improvement
- Durable: The therapy effect can last 6-15 months in patients who respond.
- Safe: Excellent safety profile with minimal adverse effects and no significant contraindications.
- Sustainable: Safe for repeated use, allowing ongoing symptom management.
- Cost-Effective: Reduces overall healthcare costs by delaying or avoiding surgery.
- Patient-Centered: Enhances quality of life, offering patients to try another option besides medication or surgery to maintain activity levels.
As medical professionals, integrating HA injections into our treatment protocols offers a valuable option for patients seeking alternatives to surgery, potentially improving patient outcomes safely and reducing overall healthcare costs.
This blog from Arthur Musculoskeletal Health is a series of free online medical education posts dedicated to providing online primary care education for physicians, nurse practitioners, physician assistants, residents, and medical students. This blog is not intended to be medical advice for patients; please see your legally qualified medical practioner for your medical condition(s). The information provided is for general and informational purposes only and is not meant to diagnose, treat, or prevent any medical condition. The post information is stated in my individual capacity, and not as a part of my duties at my hospital or academic institutions.
References
- Khan M, Adili A, Winemaker M, Bhandari M. Management of knee osteoarthritis in younger patients. CMAJ. 2018;190(3). doi:10.1503/cmaj.170696.
- da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017 Jul 8;390(10090):e21-e33. doi: 10.1016/S0140-6736(17)31744-0. PMID: 28699595.
- Weng, Goh SL, Wu J, Persson MSM, Wei J, Sarmanova A, Li X, Hall M, Doherty M, Jiang T, Zeng C, Lei G, Zhang W. Comparative efficacy of exercise therapy and oral non-steroidal anti-inflammatory drugs and paracetamol for knee or hip osteoarthritis: a network meta-analysis of randomised controlled trials. Br J Sports Med. 2023 Aug;57(15):990-996. doi: 10.1136/bjsports-2022-105898. Epub 2023 Jan 2. PMID: 36593092; PMCID: PMC10423468.
- Holden A, Murphy M, Simkins J, et al. Knee braces for knee osteoarthritis: A scoping review and narrative synthesis of interventions in randomized controlled trials. Osteoarthritis Cartilage. 2024. doi:10.1016/j.joca.2024.08.010.
- Lindblad AJ, McCormack J, Korownyk CS, et al. PEER simplified decision aid: osteoarthritis treatment options in primary care. Can Fam Physician. 2020;66:19.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015 Oct 26;16:321. doi: 10.1186/s12891-015-0775-z.
- Bisicchia S, Tudisco C. Hyaluronic acid vs corticosteroids in symptomatic knee osteoarthritis: a mini-review of the literature. Clin Cases Miner Bone Metab. 2017 May-Aug;14(2):182-185. doi: 10.11138/ccmbm/2017.14.1.182. Epub 2017 Oct 25. PMID: 29263730; PMCID: PMC5726206.
- Trojian TH, Concoff AL, Joy SM, Hatzenbuehler JR, Saulsberry WJ, Coleman CI. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016 Jan;50(2):84-92. doi: 10.1136/bjsports-2015-095683. PMID: 26729890.
- Phillips M, Vannabouathong C, Devji T, Patel R, Gomes Z, Patel A, Dixon M, Bhandari M. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020 Sep;28(9):3031-3039. doi: 10.1007/s00167-019-05763-1. Epub 2020 Jan 3. PMID: 31897550; PMCID: PMC7471203.
- Campbell KA, Erickson BJ, Saltzman BM, Mascarenhas R, Bach BR Jr, Cole BJ, Verma NN. Is Local Viscosupplementation Injection Clinically Superior to Other Therapies in the Treatment of Osteoarthritis of the Knee: A Systematic Review of Overlapping Meta-analyses. Arthroscopy. 2015 Oct;31(10):2036-45.e14. doi: 10.1016/j.arthro.2015.03.030. Epub 2015 May 19. PMID: 25998016.
- Vannabouathong, C., Bhandari, M., Bedi, A., Khanna, V., Yung, P., Shetty, V., & Khan, M. (2018). Nonoperative Treatments for Knee Osteoarthritis: An Evaluation of Treatment Characteristics and the Intra-Articular Placebo Effect: A Systematic Review. JBJS Reviews., 6(7). https://doi.org/10.2106/JBJS.RVW.17.00167
- Banuru RR, Osani M, Vaysbrot EE, McAlindon TE. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis Cartilage 2016;24:2022-41.
- McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017;317(19):1967-1975. doi:10.100 /jama.2017.5283
- Phillips M, Bhandari M, Grant J, Bedi A, Trojian T, Johnson A, Schemitsch E A Systematic Review of Current Clinical Practice Guidelines on Intra-articular Hyaluronic Acid, Corticosteroid, and Platelet-Rich Plasma Injection for Knee Osteoarthritis: An International Perspe tive. Orthop J Sports Med. 2021 Aug 31;9(8):23259671211030272. doi: 10.1177/23259671211030272. PMID: 34485586; PMCID: PMC8414628.
- Jüni P, Hari R, Rutjes AWS, Fischer R, Silletta MG, Reichenbach S, da Costa BR. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No.: CD005328. DOI: 10.1002 14651858.CD005328.pub3
- Kamel SI, Rosas HG, Gorbachova T. Local and systemic Side Effects of Corticosteroid Injections for Musculoskeletal Indications. AJR Am J Roetgenol. 2023;222(3). doi:10.2214/AJR.23.30458
- Kim, Y.M., Joo, Y.B. & Song, JH. Preoperative intra-articular steroid injections within 3 months increase the risk of periprosthetic joint infection in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 18, 148 (2023).
- Phillips M, Bhandari M, Grant J, et al. A Systematic Review of Current Clinical Practice Guidelines on Intra-articular Hyaluronic Acid, Corticosteroid, and Platelet-Rich Plasma Injection for Knee Osteoarthritis: An International Perspective. Orthopaedic Journal of Sports Medicine. 2021;9(8). doi:10.1177/23259671211030272
- Hummer, C.D., Angst, F., Ngai, W. et al. High molecular weight Intraarticular hyaluronic acid for the treatment of knee osteoarthritis: a network meta-analysis. BMC Musculoskelet Disord 21, 702 (2020). https://doi.org/10.1186/s12891-020-03729-w
- Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162(1): 46–54.
- Shan, Leonard, et al. “Intermediate and long-term quality of life after total knee replacement: a systematic review and meta-analysis.” JBJS 97.2 (2015): 156-168.
- Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, Beard DJ. Knee replacement. Lancet. 2012 Apr 7;379(9823):1331-40. doi: 10.1016/S0140-6736(11)60752-6. Epub 2012 Mar 6. PMID: 22398175.
- Papakostidis, C., Giannoudis, P.V., Watson, J.T. et al. Serious adverse events and 30-day hospital readmission rate following elective total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 16, 23 (2021). https:// oi.org/ 0.1186/s13018-021-02358-w
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron K . Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63. doi:10.1007/s11999-009-1119-9.
- Parvizi, J., Nunley, R.M., Berend, K.R. et al. High Level of Residual Symptoms in Young Patients After Total Knee Arthroplasty. Clin Orthop Relat Res 472, 133–137 (2014). https://doi.org/10. 007/s11999-013-3229-7
- Skou ST, Roos EM, Laursen MB, et al. Total knee replacement and non-surgical treatment of knee osteoarthritis: 2-year outcome from two parallel randomized controlled trials. Osteoarthritis Cartilage. 2018;26(9):1170-1180. doi:10.1016/j.joca.2018.04.014.
- Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther. 2010;40(9):559-567. doi:10.2519/jospt.2010.3317.
- Altman R, Fredericson M, Bhattacharyya SK, Bisson B, Abbott T, Yadalam S, Kim M. Association between Hyaluronic Acid Injections and Time-to-Total Knee Replacement Surgery. J Knee Surg. 2016 Oct;29(7):564-570. doi: 10.1055/s-0035-1568992. Epub 2015 Dec 7. PMID: 26641076.
- Carney G, Harrison A, Fitzpatrick J. Long-Term Outcome Measures of Repeated Non-Animal Stabilized Hyaluronic Acid (Durolane) Injections in Osteoarthritis: A 6-Year Cohort Study with 623 Consecutive Patients. Open Access Rheumatol. 2021 Sep 18;13:285-292. doi: 10.2147/OARRR.S331562. PMID: 34566438; PMCID: PMC8457651.
- Dasa V, Lim S, Heeckt P. Real-World Evidence for Safety and Effectiveness of Repeated Courses of Hyaluronic Acid Injections on the Time to Knee Replacement Surgery. Am J Orthop (Belle Mead NJ). 2018 Jul;47(7). doi: 10.12788/ajo.2018.0058. PMID: 30075038.
- Bhadra AK, Altman R, Dasa V, et al. Appropriate Use Criteria for Hyaluronic Acid in the Treatment of Knee Osteoarthritis in the United States. CARTILAGE. 2017;8(3):234-254. doi:10.1177/1947603516662503
- Oo WM, Linklater J, Siddiq MAB, Fu K, Hunter DJ. Comparison of ultrasound guidance with landmark guidance for symptomatic benefits in knee, hip and hand osteoarthritis: Systematic review and meta-analysis of randomised controlled trials. Australas J Ultrasound Med 2024; 27: 97–105.
- Hangody L, Szody R, Lukasik P, Zgadzaj W, Lénárt E, Dokoupilova E, Bichovsk D, Berta A, Vasarhelyi G, Ficzere A, Hangody G, Stevens G, Szendroi M. Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial. Cartilage. 2018 Jul;9(3):276-283. doi: 10.1177/1947603517703732. Epub 2017 May 23. PMID: 28535076; PMCID: PMC6042027.
- Brignardello-Petersen R, Guyatt GH, Buchbinder R, et al. Knee arthroscopy versus conservative management in patients with degenerative knee disease: a systematic review BMJ Open 2017; 7:e016114. doi: 10.1136/bmjopen-2017-016114
- Phillips M, Bhandari M, Grant J, Bedi A, Trojian T, Johnson A, Schemitsch E. A Systematic Review of Current Clinical Practice Guidelines on Intra-articular Hyaluronic Acid, Corticosteroid, and Platelet-Rich Plasma Injection for Knee Osteoarthritis: An International Perspective. Orthop J Sports Med. 2021 Aug 31;9(8):23259671211030272. doi: 10.1177/23259671211030272. PMID: 34485586; PMCID: PMC8414628.
- Conrozier T, Raman R, Chevalier X, et al. Viscosupplementation for the treatment of osteoarthritis. The contribution of EUROVISCO group. Therapeutic Advances in Musculoskeletal Disease. 2021;13. doi:10.1177/1759720X211018605
- Arthroscopy Association of Canada; Kopka M, Sheehan B, Degen R, Wong I, Hiemstra L, Ayeni O, Getgood A, Beavis C, Volesky M, Outerbridge R, Matache B. Arthroscopy Association of Canada Position Statement on Intra-articular Injections for Knee Osteoarthritis. Orthop J Sports Med. 2019 Jul 19;7(7):2325967119860110. doi: 10.1177/2325967119860110. PMID: 31367647; PMCID: PMC6643188.
- Altman RD, Ike RW, Hamburger MI, McLain DA, Daley MJ, Adamson TC III. Missing the mark? American College of Rheumatology 2019 guidelines for intraarticular hyaluronic acid injection and osteoarthritis knee pain. J Rheumatol. 2022;49(8):9 8-960. doi:10.3899/jrheum.220125.
- Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Therapeutic Advances in Musculoskeletal Disease. 2017;9(9):231-246. doi:10.1177/1759720X17729641
- Ranawat, A., Guo, K., Phillips, M. et al. Health Economic Assessments of Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review. Adv Ther 41, 65–81 (2024). https://doi.org/10.1007/s12325-023-02691-y
- Berton A, Longo UG, Candela V, Greco F, Martina FM, Quattrocchi CC, Denaro V. Quantitative Evaluation of Meniscal Healing Process of Degenerative Meniscus Lesions Treated with Hyaluronic Acid: A Clinical and MRI Study. J Clin Med. 2020 Jul 17;9(7):2280. doi: 10.3390/jcm9072280. PMID: 32709084; PMCID: PMC7408658.
- Zorzi C, Rigotti S, Screpis D, Giordan N, Piovan G. A new hydrogel for the conservative treatment of meniscal lesions: a randomized controlled study. Joints. 2016 Jan 28;3(3):136-45. doi: 10.11138/jts/2015.3.3.136. PMID: 26889470; PMCID: PMC4732780.
- Dong Z, Huang L, Wu G, Li P, Wei X. Efficacy and safety of arthroscopic surgery combined with hyaluronic acid for meniscal injuries: A systematic review and meta-analysis of randomized controlled studies. Journal of Orthopaedic Surgery. 2023;31(1). doi:10.1177/10225536231156699
- Siemieniuk R A C, Harris I A, Agoritsas T, Poolman R W, Brignardello-Petersen R, Van de Velde S et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline BMJ 2017; 357: j1982 doi:10.1136/bmj.j1982
#KneeOsteoarthritis #HyaluronicAcid #HAInjections #KneePainRelief #JointHealth #Viscosupplementation #NonSurgicalTreatment #GlucocorticoidInjections #TotalKneeArthroplasty #PatientCare #SportsMedicine #Chondroprotection #PainManagement #OrthopedicHealth #HealthInnovation #ArthritisTreatment #KneeBracing #ExerciseTherapy #MetaAnalysis #PatientSatisfaction #ChronicPainManagement
